Module 2: Lipids
These free OCR A Level Biology Lipids revision notes have been written for specification points 2.1.2(h), 2.1.2(i) and 2.1.2(j).
Lipids
Lipids are a group of molecules with a wide variety of structures and functions in organisms, but most importantly, they are used in cell plasma membranes and for energy storage and thermal insulation.
| Lipid Type | Components | Ester bonds? | Image |
|---|---|---|---|
| Triglyceride | Glycerol + 3 fatty acids | Yes |  |
| Phospholipid | Glycerol + 2 fatty acids + phosphate group | Yes | 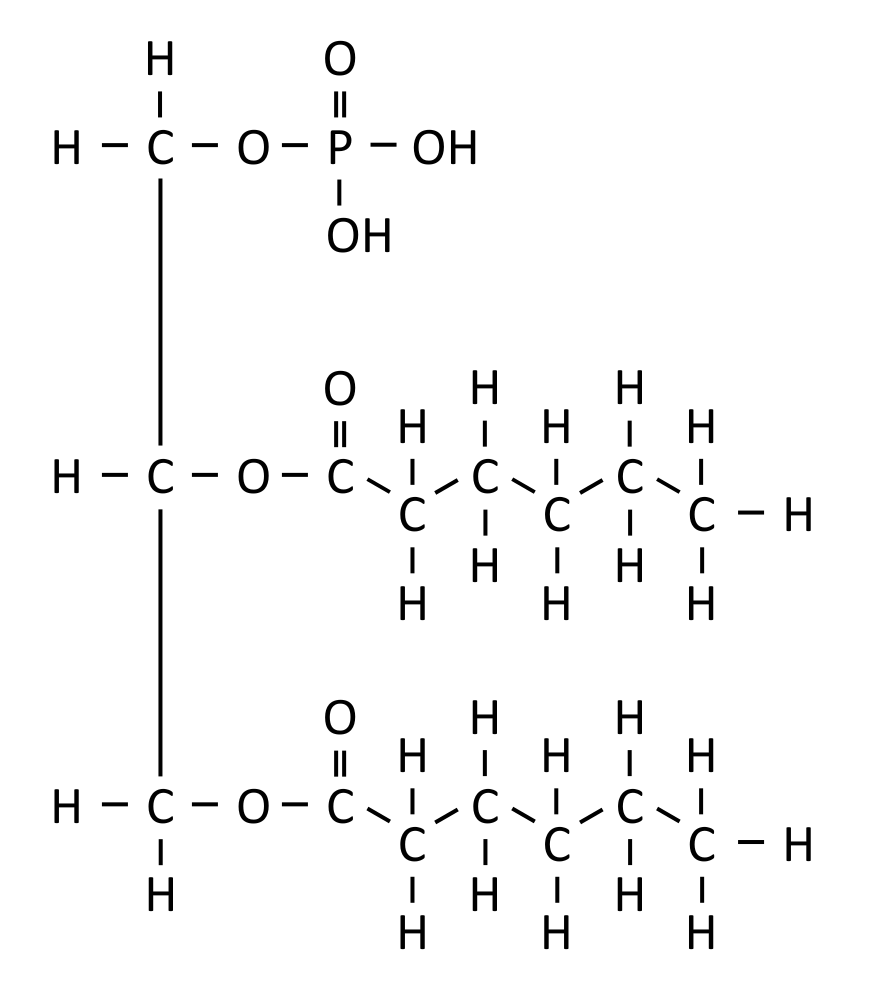 |
| Steroid* | Four fused carbon rings | No | 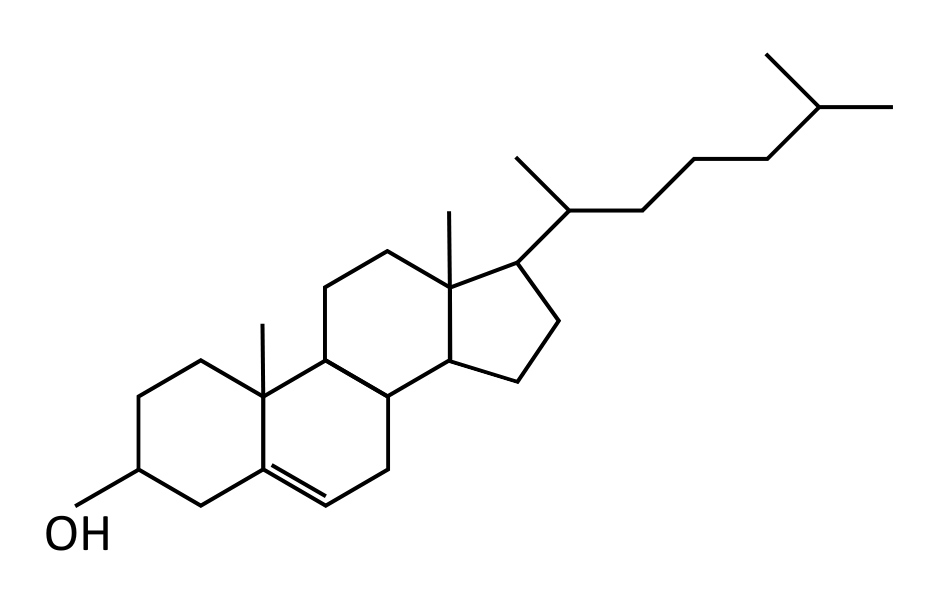 |
*The OCR A level Biology specification uses cholesterol to represent all steroids.
Lipids are (mostly) non-polar, so they are (usually) insoluble in water; however, they do dissolve in alcohol.
Fatty acids
Fatty acids are one of the components that make up triglycerides and phospholipids, the other being glycerol.
Fatty acids are not lipids, but are instead used to make them.
Fatty acids are long-chain hydrocarbons with a carboxyl group on one end.
They can either be saturated or unsaturated.
Saturated fatty acids have no C=C bonds, and are a straight chain.
Unsaturated fatty acids have at least one C=C bond, which causes a kink in the chain.
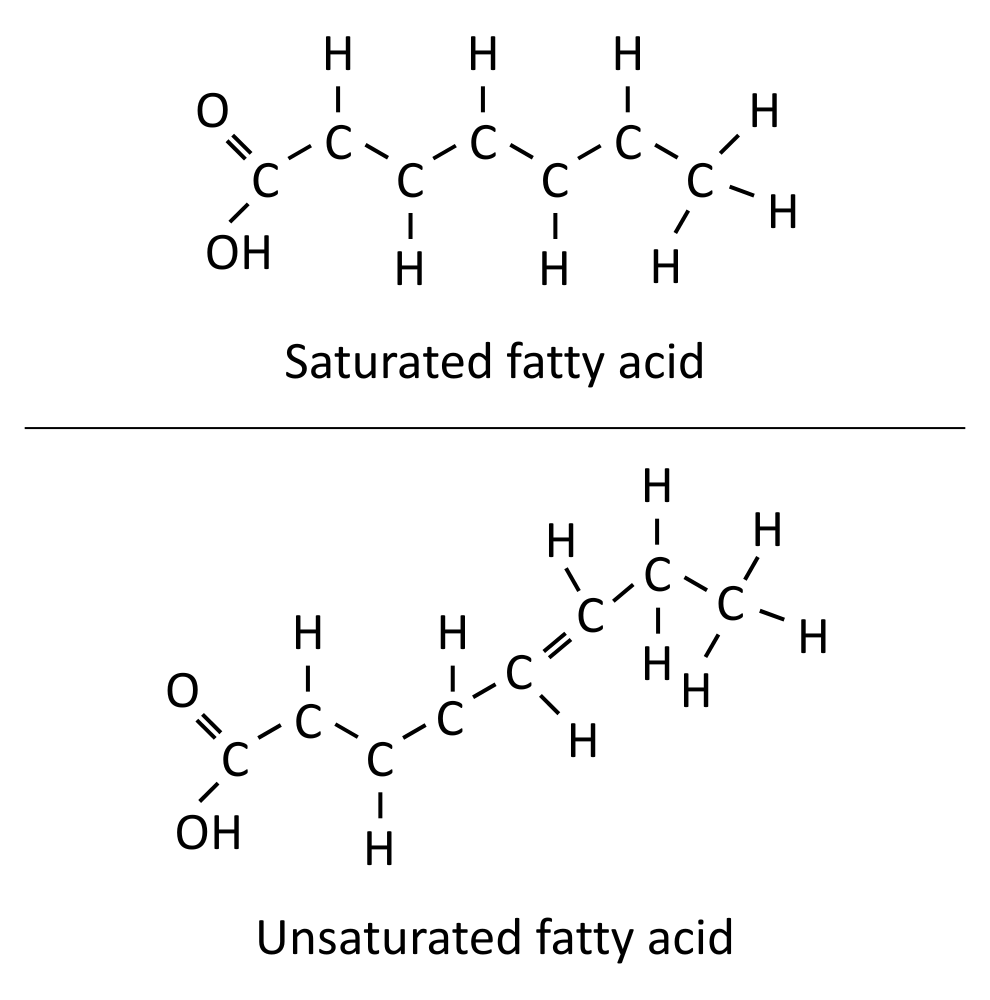
The more C=C bonds an unsaturated fatty acid has, the lower its melting point.
Triglyceride Synthesis
Triglycerides are made by joining glycerol to three fatty acids using ester bonds.
Ester bonds are formed in condensation reactions between the hydroxyl (–OH) group on glycerol and the carboxyl group on a fatty acid. This produces a molecule of water as waste.
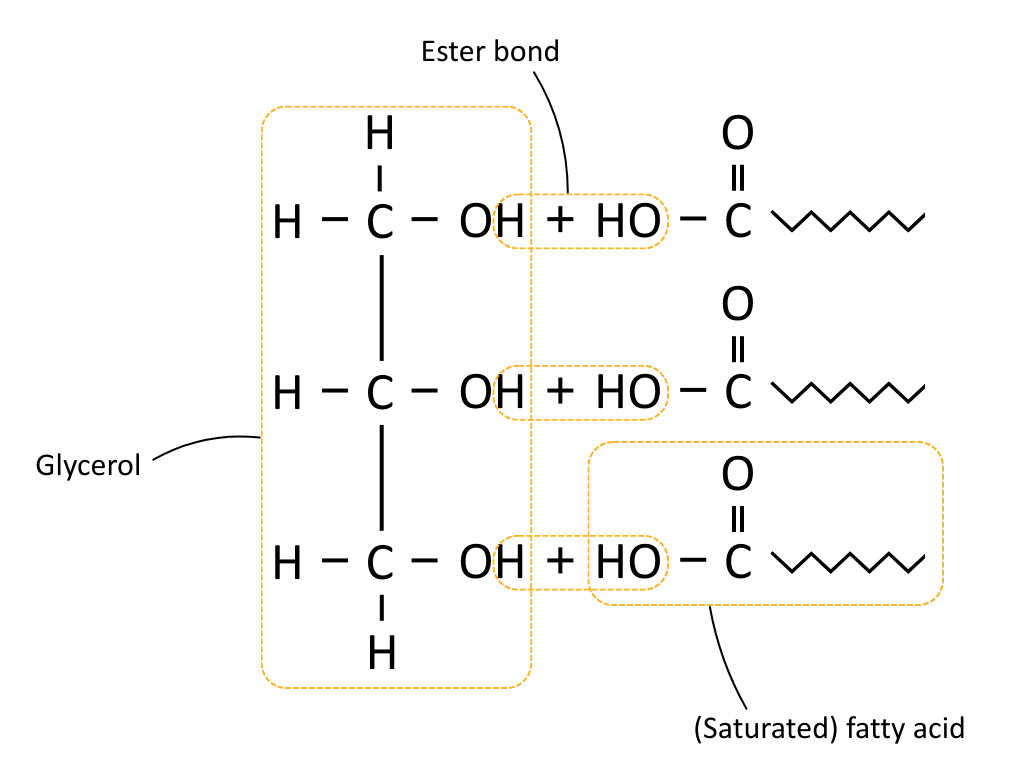
When lipids are hydrolysed (digested), enzymes such as lipase break the ester bonds using water, releasing glycerol and fatty acids.
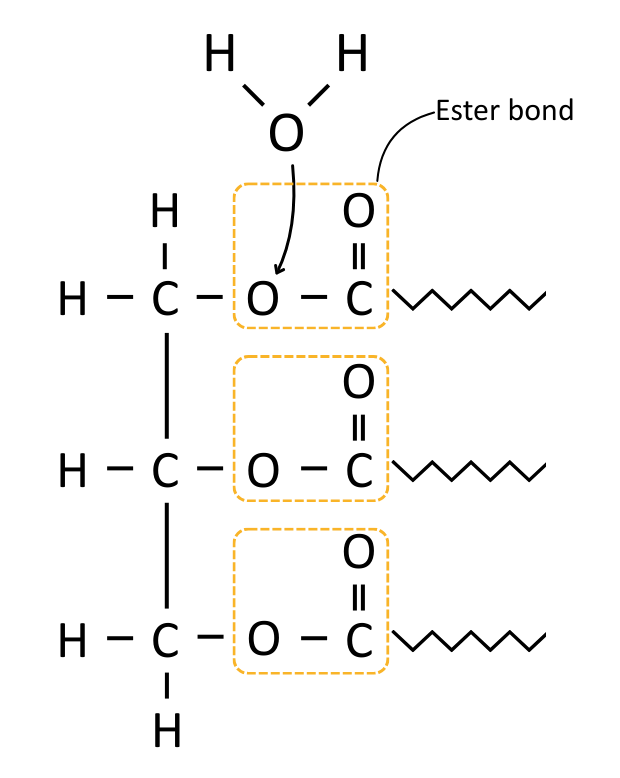
Biological Functions of Lipids
The table below outlines how structure relates to the function of each lipid type’s use:
| Lipid Type | Use(s) | How Structure Supports Function |
|---|---|---|
| Triglyceride | Energy storage, insulation, protection |
Hydrophobic and compact. Stores large amounts of energy. |
| Phospholipid | Forms membranes, emulsifier |
Amphipathic: hydrophilic phosphate head and hydrophobic fatty acid tails allow bilayer formation and emulsification of fats in water. |
| Steroid | Hormones, membrane stability |
Small, flat, mostly hydrophobic molecules, so they can diffuse through membranes. Cholesterol fits between phospholipid tails to stabilise membranes. |
| Wax | Waterproofing and protection |
Hydrophobic and solid at room temperature, so it forms protective barriers against water loss or microbial entry. |
Lipid insolubility is important because they do not affect water potential, allowing energy-rich molecules to be stored without significant changes in osmotic balance.
