Module 2: Enzymes
These free OCR A Level Biology Enzymes revision notes have been written for specification points 2.1.4(a), 2.1.4(b), 2.1.4(c) and 2.1.4(f).
Enzymes
Enzymes are globular proteins with a specific active site (determined by their tertiary structure) that catalyse biochemical reactions by lowering the activation energy required.
This active site is complementary (specific) to a substrate with a specific shape (or at least substrates similar enough to fit into the active site). This is known as ‘enzyme specificity’.
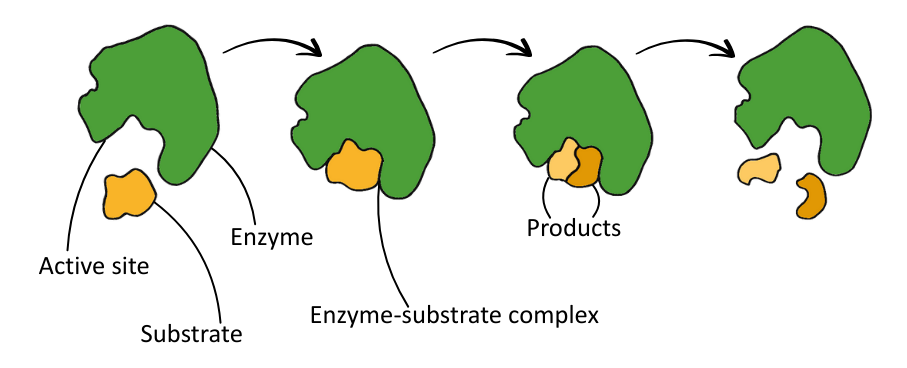
Enzymes can break apart molecules (catabolism) or join them together (anabolism).
The two examples you need to know about are amylase (extracellular) and catalase (intracellular):
| Amylase (humans) | Catalase (humans) | |
|---|---|---|
| Location | Extracellular | Intracellular |
| Function | Breaks down starch into maltose during digestion | Breaks down hydrogen peroxide (H₂O₂) into water and oxygen; protects cells from oxidative damage |
| Location (of work) | Mouth and small intestine | Found inside most cells |
| Produced By | Salivary glands and pancreatic cells | Most aerobic cells High levels in liver and white blood cells |
| Substrate | Starch | Hydrogen peroxide (H₂O₂) |
| Products | Maltose | Water and oxygen |
| Biological Role | Digestive enzyme | Protects against oxidative damage, used to kill pathogens (with oxidative damage) |
| Optimal pH | ~7 (salivary) ~6.7–7.0 (pancreatic) | ~7 |
| Optimal Temperature | ~37 °C | ~45 °C |
Enzyme Action: Lock and Key, and Induced Fit
The table below summarises the two models of enzyme action:
| Model | Description |
|---|---|
| Lock and Key | The active site is a perfect fit for the substrate (they are complementary); like a key fitting into a lock. |
| Induced Fit | The active site undergoes a conformational change (changes shape slightly) to better fit the substrate when it binds. This improves binding and catalysis. |
These two models are typically discussed separately and compared, but the induced-fit hypothesis builds upon the lock-and-key hypothesis to improve it.
The table below summarises the process of enzyme action:
| Stage | Description |
|---|---|
| 1. Enzyme + Substrate (E+S) | Substrate collides with the enzyme’s active site. |
| 2. Enzyme–Substrate Complex (ESC) | Substrate binds to the enzyme’s active site with temporary hydrogen bonds, ionic attractions, hydrophobic interactions and van der Waals forces. |
| 3. Enzyme–Product Complex (EPC) | The enzyme catalyses (anabolism or catabolism) the conversion of substrate into product. |
| 4. Enzyme + Product (E+P) | The product is released from the active site. |
Significance of Enzymes
The table below outlines some examples of the structural and functional importance of enzymes:
| Level | Structure | Function |
|---|---|---|
| Molecular | Build proteins, nucleic acids, and membranes | Catalyses essential chemical reactions |
| Cellular | Affects cell wall, cytoskeleton, and organelle shape | Controls respiration, division, and signalling |
| Tissue/Organ | Shapes connective tissue, cartilage, etc. | Supports digestion, immunity, and nerve function |
| Organism | Developmental patterning | Growth, anatomical development, and repair |
Cofactors
Cofactors are non-protein substances that help or enable an enzyme’s function by making it easier for a substrate to bind to the active site.
They do this by:
- Stabilising charge distribution
- Helping substrates bind
- Directly participating in the reaction
Cofactors typically bind to the enzyme’s active site, or near it, either temporarily or permanently.
The table below outlines each type:
| Type | Description |
|---|---|
| Cofactor | Inorganic ions that temporarily bind to the enzyme to aid its function. |
| Cosubstrate | Organic molecules that act like substrates to complete the complementary shape. |
| Coenzyme | Organic, non-protein molecules derived from vitamins that temporarily bind to the enzyme’s active site. |
| Prosthetic Group | Non-protein group permanently bound to the enzyme (covalently). Essential for enzyme function. |
Coenzymes and Vitamins
Organic coenzymes are usually derived from vitamins, and a deficiency in one or more of these impacts metabolic function due to the effects of poor enzyme activity.
The table below outlines these different vitamins:
| Vitamin | Vitamin Name | Coenzyme Derived | Human Deficiency Disease |
|---|---|---|---|
| B₁₂ | Cobalamin | Cobalamin coenzymes | Pernicious anaemia (progressive, fatal anaemia) |
| B₉ | Folic acid (Folate) | Tetrahydrofolate | Megaloblastic anaemia (large irregularly shaped erythrocytes) |
| B₃ | Niacin (Nicotinamide) | NAD, NADP | Pellagra (dementia, dermatitis, diarrhoea) |
| B₅ | Pantothenic acid | Coenzyme A | Elevated blood-plasma triglyceride levels |
| B₁ | Thiamine | Thiamine pyrophosphate (TPP) | Beriberi (heart failure, irregular heartbeat, mental confusion, muscular weakness, paralysis) |
Pearson’s 2015 edition textbook for OCR A-level Biology incorrectly labels pantothenate as vitamin B₆, when it should be vitamin B₅ (pantothenic acid), and also (inconsistently) omits specifying that folic acid is vitamin B₉.
Inhibitors
Inhibitors are substances that reduce the rate of enzyme-controlled reactions.
Inhibitors work by interfering with the enzyme’s ability to form enzyme-substrate complexes.
Inhibition is defined by:
- Reversibility – Can the enzyme function normally ‘if’ the inhibitor unbinds?
- Competition – Does the inhibitor compete with the substrate for the active site?
- Binding site – Does the inhibitor bind to the active site, or the allosteric site (an external region on the enzyme)?
The two types of inhibitor are competitive and non-competitive:
| Type | Binding Site | Substrate Competition | Reversible? |
|---|---|---|---|
| Competitive | Active site | Yes | Yes* |
| Non-Competitive | Allosteric site | No | Varies |
*Usually
The diagram below shows the binding action of competitive and non-competitive inhibitors.
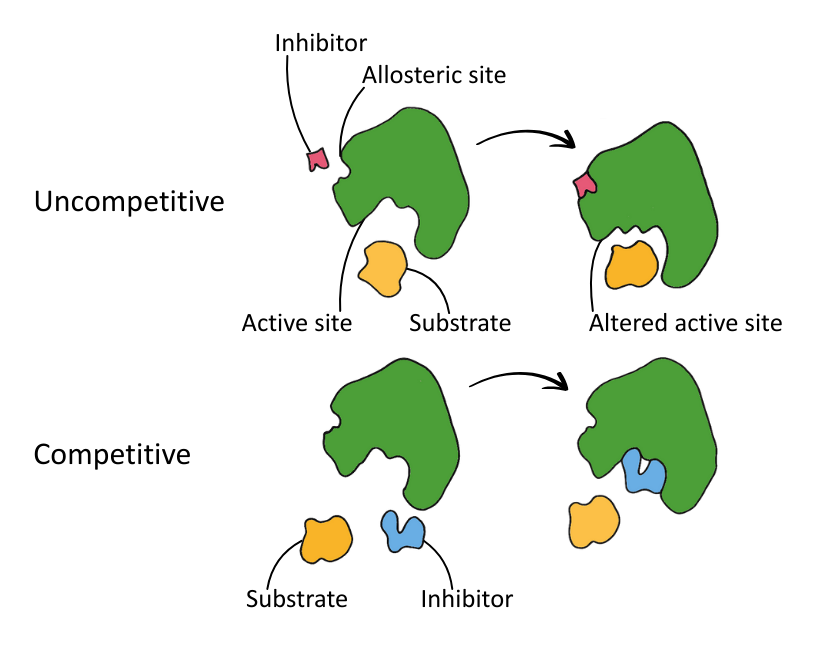
The graph below shows the rates of reaction observed when different types of inhibitors are added to a reaction catalysed by enzymes: