Module 2: Chromatography
These free OCR A Level Biology Chromatography revision notes have been written for specification points 2.1.2(s.i) and (s.ii).
Chromatography
Chromatography is a technique used to separate and identify biological molecules.
Substances which are highly soluble and have a low affinity towards the material will travel at a greater rate than those with a lower solubility and/or higher affinity.
Larger molecules also move more slowly than smaller molecules (of the same solubility and affinity).
The two components used are:
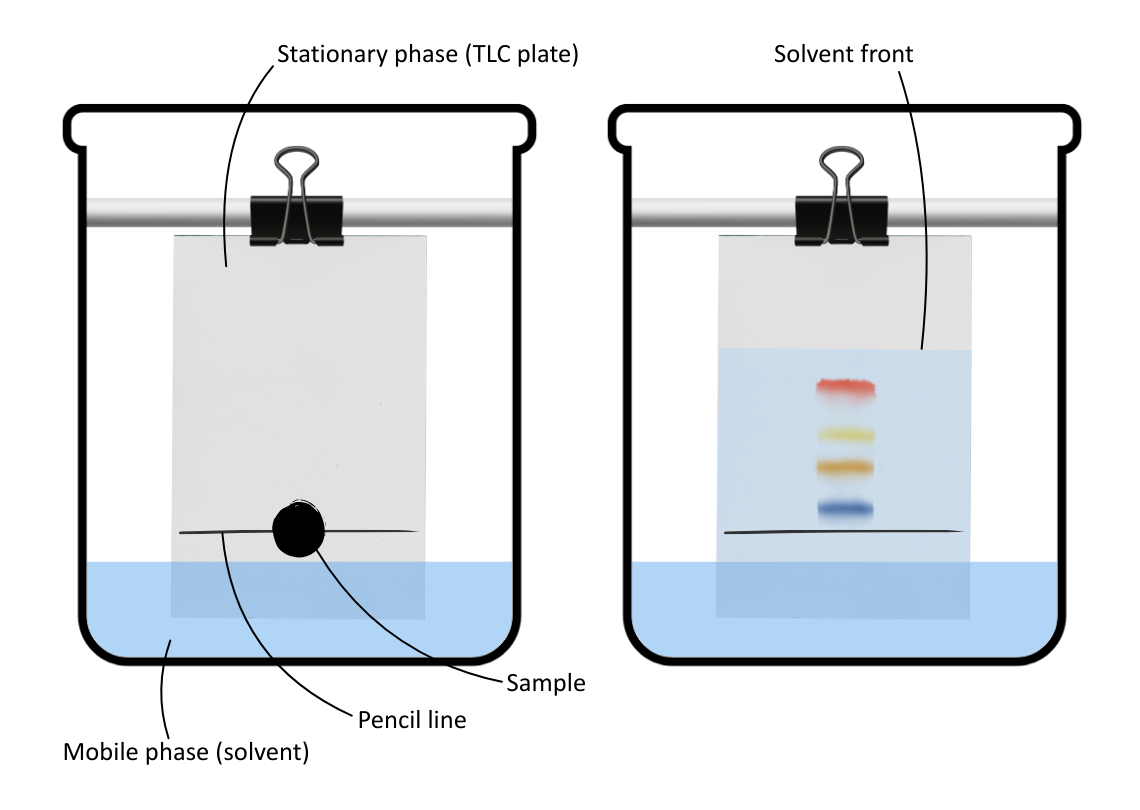
- Stationary phase: A paper (cellulose) or TLC plate (plastic covered in silica or aluminium hydroxide).
- Mobile phase: A solvent in which the biological molecule is dissolved.
The table below outlines example molecules that can be separated:
| Molecule Separated | Use in Biology |
|---|---|
| Amino acids | Identify components in proteins (e.g. protein digestion analysis) |
| Carbohydrates (sugars) | Detect the presence and type of sugars in a sample |
| Vitamins | Separate and identify different vitamins in food samples |
| Nucleic acids | Used in genetic research to analyse DNA/RNA fragments |
| Hormones/Drugs | Athletic anti-doping tests |
Practical method:
- A sample is spotted onto a pencil line (used to measure how far substances have travelled) on chromatography paper or the TLC plate.
- The plate/paper is placed in a solvent (the mobile phase) with the pencil line above the solvent.
- The solvent moves up the stationary phase via capillary action, coming into contact and dissolving the sample molecules.
- Sample molecules move upwards and separate out, based on their affinity and solubility.
- Once the solvent front reaches the top, the chromatogram is removed and dried.
- Calculate each molecule’s Rf value (how much it dissolves into the mobile phase.
Calculating Rf Values:
Rf = Distance moved by the solute ÷ Distance moved by the solvent front
- Distance moved by solute: Measure from the baseline (pencil line) to the centre of the spot.
- Distance moved by solvent: Measure from the baseline to the solvent front (before it dries!).
- Compare Rf values with known standards to identify molecules.
Detecting Colourless Molecules
In GCSE practicals, coloured pigments are typically used to observe the separation of substances, but many biomolecules do not have a discernible colour.
The following treatments make them visible:
- Iodine vapour: Iodine binds to organic molecules, staining them brown.
- Ninhydrin spray: Reacts with amino acids, turning them brown or purple.
- Ultraviolet (UV) light: TLC plates may have a UV-reactive coating. Molecules block fluorescence, revealing dark spots.
