Module 4: Antibodies
These free OCR A Level Biology Antibodies revision notes have been written for specification points 4.1.1(h) and 4.1.1(i).
Antibodies
Antibodies are glycoproteins involved in the function of the immune system.
They have a quaternary structure made up of four polypeptide chains; there are two heavy chains and two light chains.
The structure of an antibody is Y-shaped, as illustrated in the diagram below:
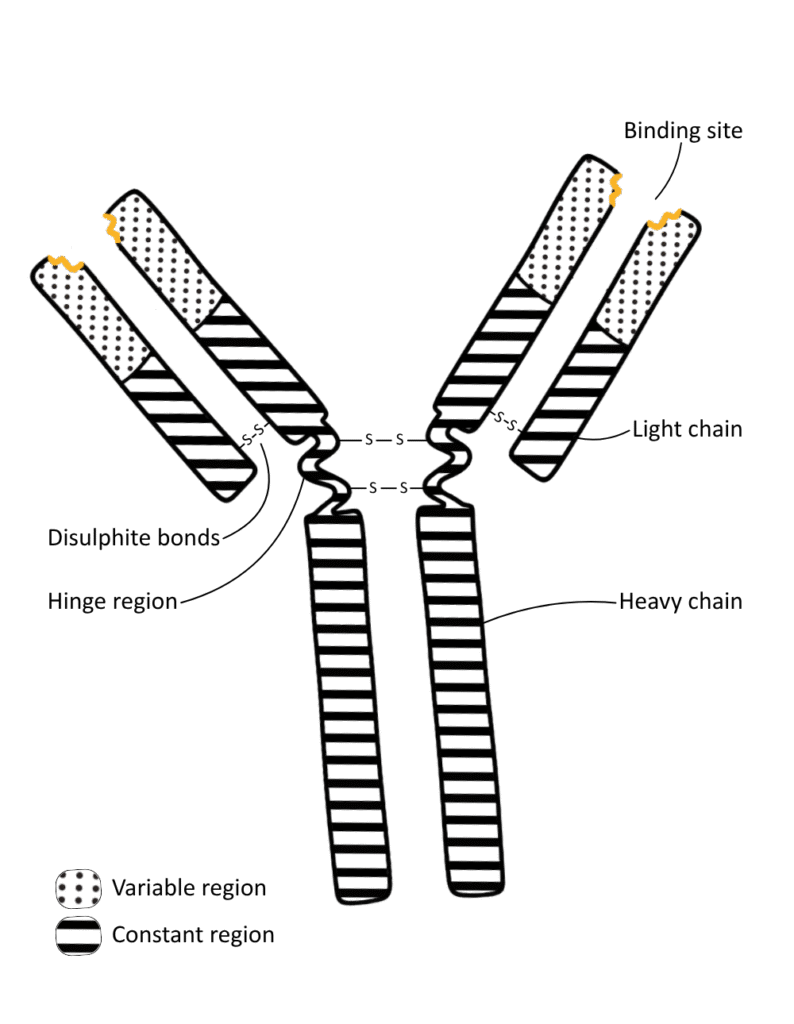
The components labelled are:
- Arms: The two branches of an antibody.
- Variable region: An area at the end of the antibody arm that differs between types of antibody; the binding site is found here.
- Binding site: A 3D area on the variable region that is complementary to one type of antigen.
- Constant region: A structure that is the same across all antibodies; it may have a receptor to help phagocytes bind to it.
- Hinge region: A flexible part of the antibody which allows more than one pathogen to be attached to (by each arm).
- Disulfide bonds: These hold the polypeptide chains together.
There are three types of antibodies, each with a different role:
- Opsonins: Bind to antigens on pathogens to make it easier for phagocytes to engulf them by providing a binding site for phagocytosis.
- Agglutinins: Bind to antigens on different pathogens so that they are clumped together (agglutinated), allowing more to be engulfed by phagocytes.
- Anti-toxins: Bind to the toxins made by pathogens, making them inactive and neutralising their harmful effects.
