Module 2: Carbohydrates
These free OCR A Level Biology Carbohydrates revision notes have been written for specification points 2.1.2(d), 2.1.2(e), 2.1.2(f) and 2.1.2(g).
Carbohydrates
Carbohydrates are a group of biological molecules that are a key source of energy and have structural roles in both animals and plants; glucose is one of the most important.
Glucose comes in two forms (isomers) called α (alpha) glucose and beta (β) glucose.
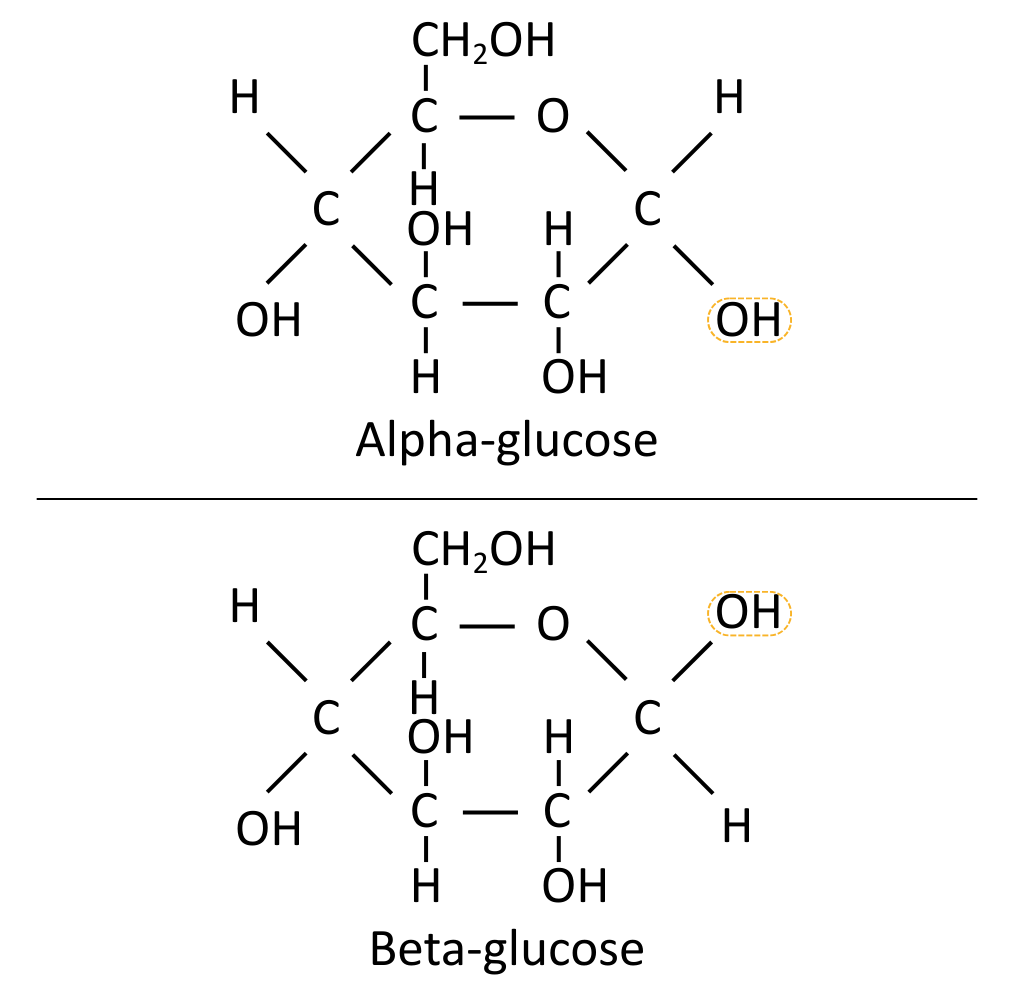
Monosaccharides
Monosaccharides are individual sugar monomers, such as glucose.
The table below outlines some of the most common monomers used to build larger carbohydrates:
| Monosaccharide | Molecular Formula | Type | Use |
|---|---|---|---|
| α-Glucose | C₆H₁₂O₆ | Hexose | Energy source and primary respiratory substrate. |
| β-Glucose | C₆H₁₂O₆ | Hexose | Energy source, a component of glycolipids and glycoproteins |
| Ribose | C₅H₁₀O₅ | Pentose | A component of nucleotides (e.g. ATP, RNA) |
All of these monosaccharide monomers are reducing, so they test positive in a Benedict’s test.
Disaccharide and Polysaccharide Formation
Carbohydrates like disaccharides and polysaccharides are made by joining monosaccharides using glycosidic bonds.
Glycosidic bonds form in condensation reactions between the hydroxyl groups of two monosaccharides, releasing a water molecule as waste.
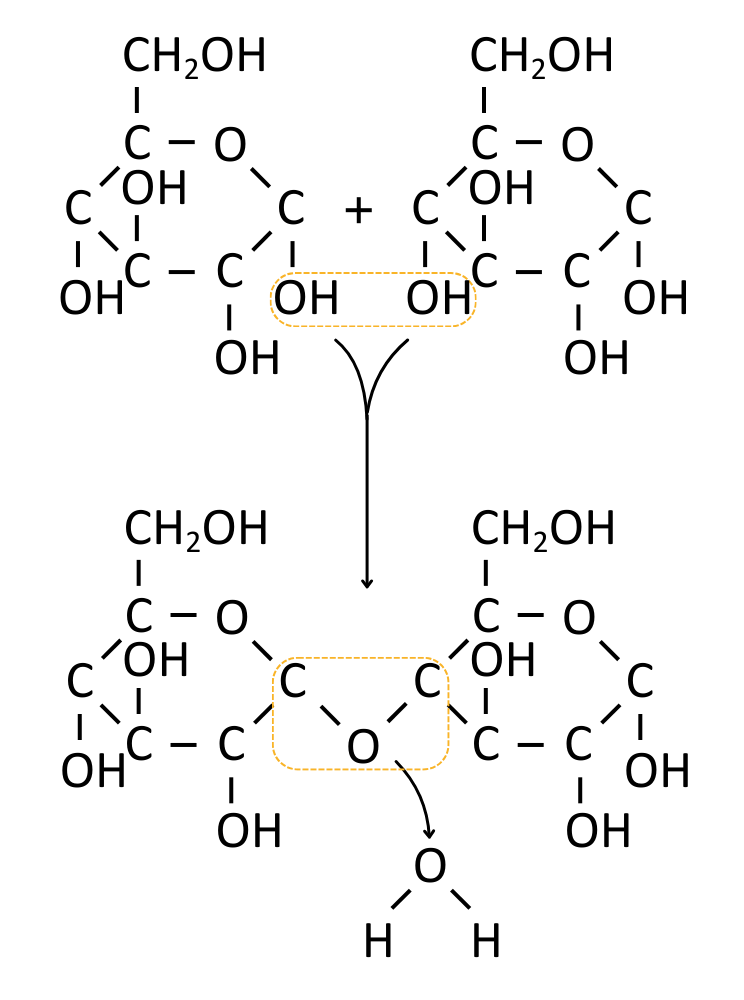
When carbohydrates are hydrolysed (digested), enzymes (e.g. amylase or maltase) break the glycosidic bonds using water, releasing smaller sugars or monosaccharides.
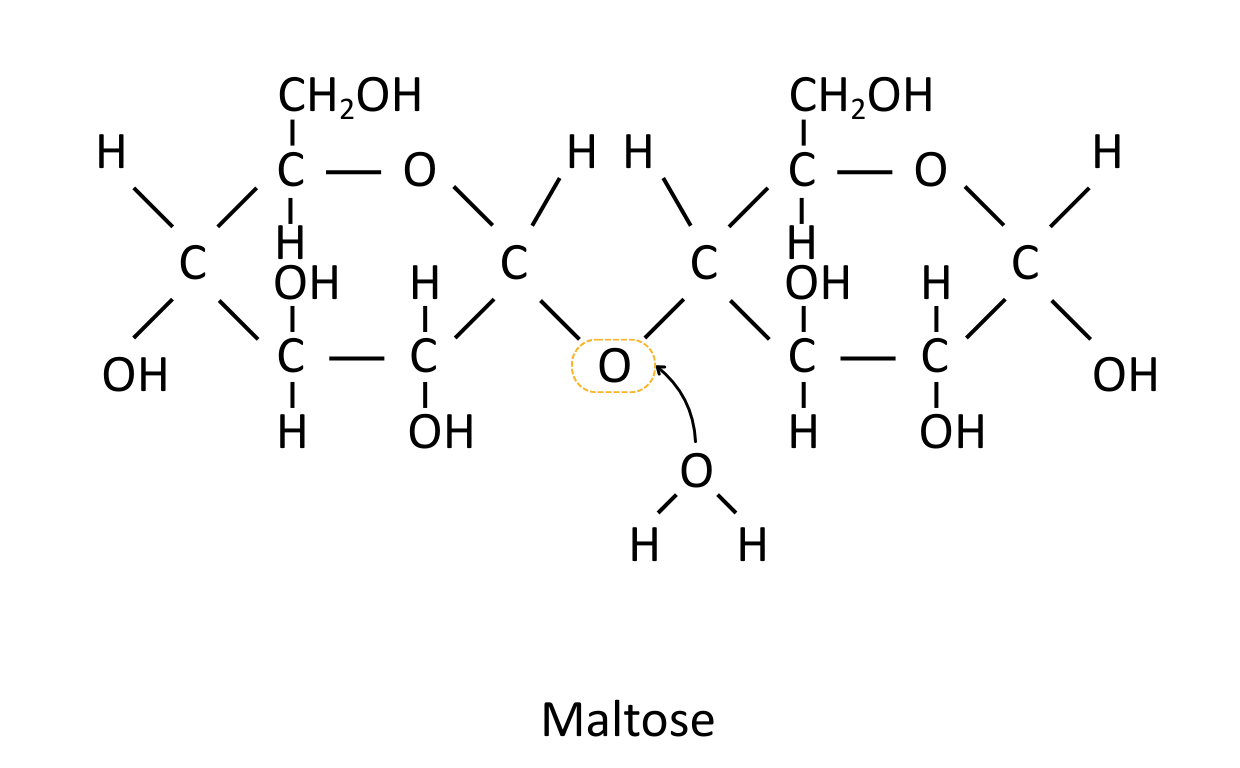
Disaccharides
Disaccharides are two sugar molecules joined together with a glycosidic bond.
The table below outlines the most common disaccharides formed from monosaccharides:
| Disaccharide | Monomers joined | Use |
|---|---|---|
| Cellobiose | β-Glucose + β-Glucose | Intermediate in cellulose breakdown |
| Lactose | α-Glucose + β-Galactose | Sugar in milk and an energy store |
| Maltose | α-Glucose + α-Glucose | Intermediate in starch digestion |
| Sucrose | α-Glucose + Fructose | Transport sugar in plants |
Of these disaccharides, only sucrose is non-reducing, so it gives a negative result in a Benedict’s test.
Polysaccharides
Polysaccharides are long chains of sugar monomers joined by glycosidic bonds.
The table below outlines the structure of the 4 main polysaccharides:
| Polysaccharide | Monomers | Image | Glycosidic Link | Structure | Compact? |
|---|---|---|---|---|---|
| Cellulose | β-Glucose + β-Glucose |
 | 1-4 | Straight chain with many hydrogen bonds between and within chains. |
 |
| Glycogen | α-Glucose + α-Glucose |
 | 1-4 and 1-6 | Coiled (less than starch) and highly branched |
 |
| Amylose | α-Glucose + α-Glucose |
 | 1-4 | Coiled into a spiral, held together by hydrogen bonds |
 |
| Amylopectin | α-Glucose + α-Glucose |
 | 1-4 and 1-6 | Coiled into a spiral held together by hydrogen bonds, but with branches (less than glycogen) |
 |
The table below outlines how structure relates to the function of each polysaccharide’s use:
| Polysaccharide | Use(s) | How Structure Supports Function |
|---|---|---|
| Cellulose | Structural support in plant cell walls | Many hydrogen bonds between fibres provide tensile strength and rigidity. |
| Glycogen | Energy storage in animals |
Compact spiral to store many glucose molecules. 1-6 glycosidic bonds create branches providing many access points for enzymes to release glucose molecules quickly. |
| Amylose | Long-term (slow-release) energy storage in plants | Compact spiral to store many glucose molecules. |
| Amylopectin | Energy storage in plants |
Compact spiral to store many glucose molecules. 1-6 glycosidic bonds create branches providing many access points for enzymes to release glucose molecules quickly. |
Polysaccharides are broken down into monomers and disaccharides during digestion by enzymes, typically to release monomers, which can then be used to release energy in respiration.
