Module 2: Water
These free OCR A Level Biology Water revision notes have been written for specification point 2.1.2(a).
The Biological Importance of Water
Water is essential to life as we know it, with unique properties arising from its structure.
Water molecules are polar, which allows them to form hydrogen bonds with each other and other important molecules.
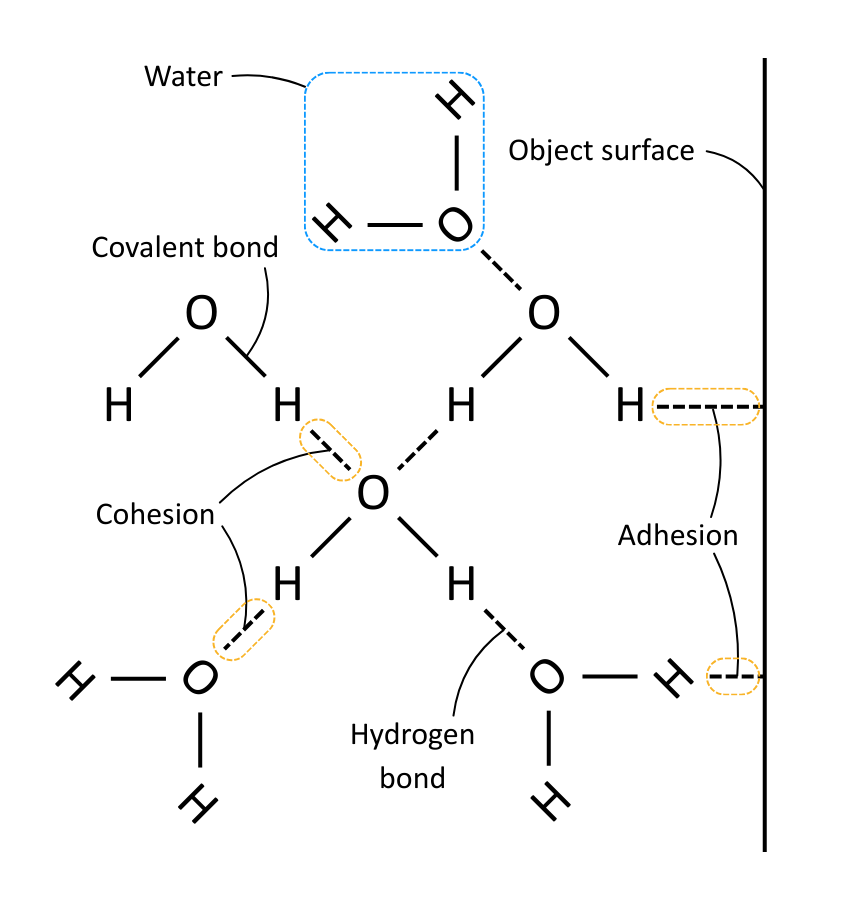
Cohesion is where water molecules form hydrogen bonds among themselves.
Adhesion is where water molecules form hydrogen bonds with other molecules.
The table below outlines the important properties of water and their importance in biology:
| Property | Description | Biological Importance |
|---|---|---|
| Density | Ice is less dense than liquid water. | Ice floats, forming an insulating layer, allowing aquatic organisms to survive in stable temperatures. |
| Solvent | Polar molecule that dissolves ionic and polar substances. | Allows solutes to dissolve in the cytoplasm. Enables transport and metabolic reactions. |
| High specific heat capacity | Absorbs lots of heat before the temperature rises due to hydrogen bonding. | Stabilises temperature in organisms and environments. |
| High latent heat of vaporisation | Requires lots of energy to evaporate. | Evaporation removes heat (e.g. sweat, transpiration). Helps cool organisms and regulate body temperature. |
| Cohesion and adhesion | Water sticks to itself (cohesion) and surfaces (adhesion). Due to hydrogen bonds. | Supports capillary action in xylem. Enables surface tension – lets small organisms walk on water. |
| Role in metabolism | Reactant in hydrolysis and photosynthesis. Product of condensation reactions and respiration. | Involved in anabolic and catabolic reactions. Medium for reactions and solvent for transport. |
